FQHC Regulatory Updates Pre and Post COVID-19
Pre-COVID-19, Community Health Centers (CHCs) faced daily challenges in order to provide the best quality care for their patients. Post-COVID, CHCs battle to overcome unfamiliar challenges while adapting to new Federal and State regulation changes. Here we will highlight resources that can help guide you through the rapidly changing Federal and State regulations.
Timeline of Federal and State Regulatory Changes
- March 6: President Trump signed into law The Coronavirus Preparedness and Response Supplemental Appropriations Act awarding $8.3 billion in emergency funding for federal agencies to respond to the COVID-19 outbreak. This package funds vaccine development, state and local governments and health policy changes.
- March 18: A $100 billion package expands unemployment insurance, paid sick leave and mandates free testing for the virus.
- March 24: The U.S. Department of Health and Human Services (HHS), through the Health Resources and Services Administration (HRSA), awarded $100 million to 1,381 health centers across the country.
- March 27: President Trump completed and signed the Coronavirus Aid, Relief, and Economic Security Act (CARES Act), releasing $2.2 trillion for regulatory relief & economic aid for individuals, state governments, businesses, and healthcare sector.
- April 8: Under the CARES Act, HRSA announced the release of more than $1.32 billion in fiscal year 2020 funding in supplemental grants for community health centers.
- April 24: A $484 billion package was signed into law for CARES Act SBA loans, HHS provider relief fund, and COVID-19 testing.
- May 7: HRSA announced the release of approximately $583 million in fiscal year 2020 Expanding Capacity for Coronavirus Testing (ECT) funding provided by the Paycheck Protection Program and Health Care Enhancement Act.
What These Changes Mean to You
If your health center was awarded the Fiscal Year 2020 CARES Act supplemental funding, this award is to be used to support health centers' ability to detect, prevent, diagnose, and treat COVID-19. The awards will also help maintain or increase health center capacity and staff levels during the pandemic public health emergency.
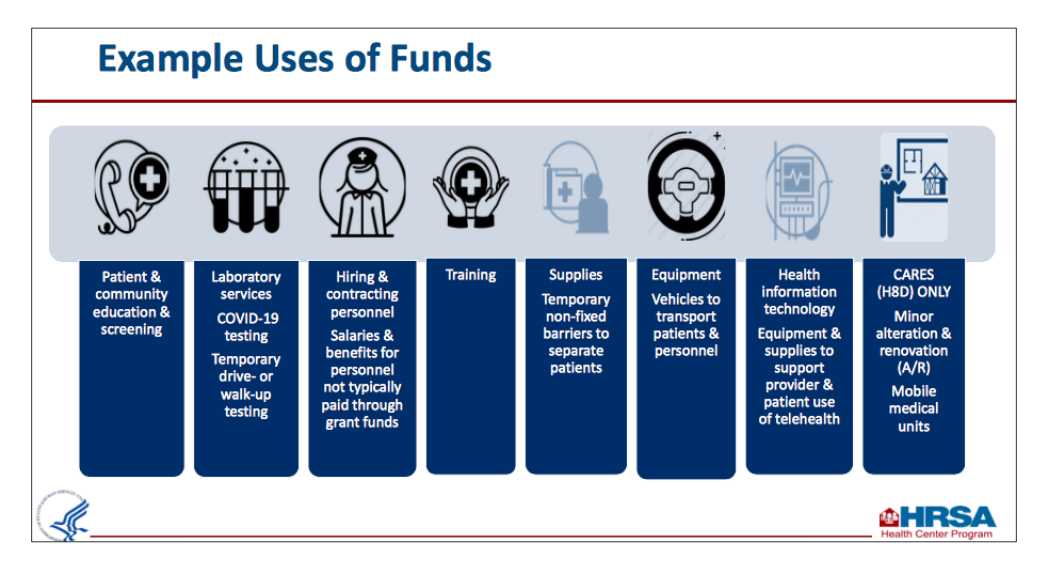
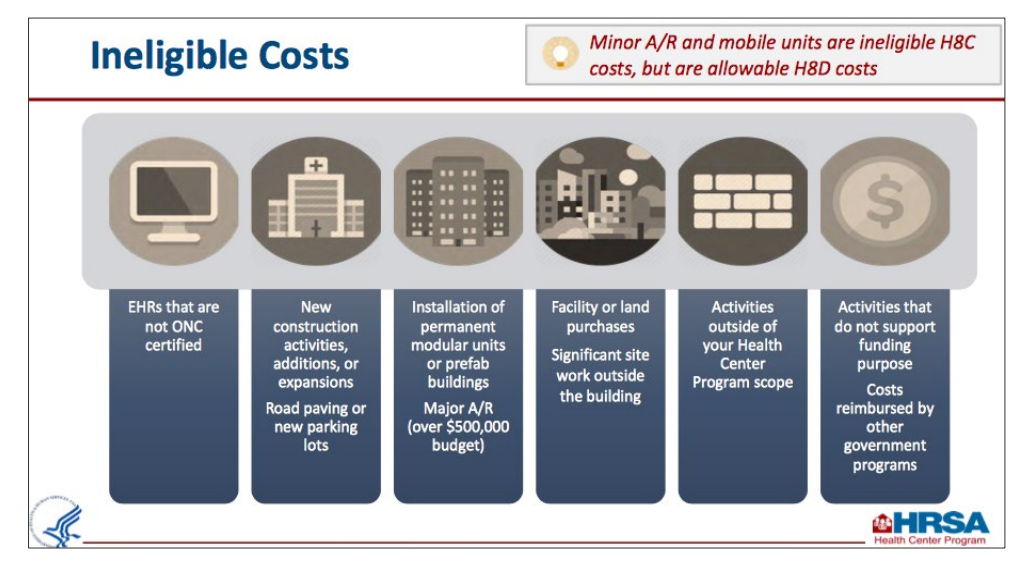
Medicare Telehealth Regulatory Changes for COVID-19
Timeline
- March 6: Phase 1 bill was signed into law and allows certain telehealth rules to be waived during a National Emergency.
- March 17: Consistent with the March 6 law and President Trump's March 13 announcement for an emergency declaration under the Stafford Act and the National Emergencies Act, Centers for Medicare and Medicaid Services (CMS) expands Medicare’s telehealth benefits under 1135 waiver authority.
- March 27: The CARES Act was signed into law adding FQHCs to the list of Medicare telehealth providers during the emergency.
- March 30: CMS announces additional regulatory actions to promote telehealth in a 221-page regulation
- April 2: Under the CARES Act, $200 Million was provided to the Federal Communications Commission (FCC) to establish a telehealth program for non-profit healthcare providers to purchase, implement, and support telehealth services.
- April 17: CMS releases Medicare telehealth guidance for FQHCs.
- April 30: CMS announces additional regulatory actions to promote telehealth in a 279-page regulation and CMS updates FQHC guidance.
Medicare Pre-COVID and Post-COVID
Images below provided by NextGen Healthcare


For more details, visit the Federal Communications Commission specifically dedicated to COVID-19 relief.
Additional resources:
