At OSIS, we are kicking off the Health Center Program Uniform Data System (UDS) reporting season with Members around our Network. As we’re preparing for crunch time, whether a seasoned veteran or it’s the first time reporting, quality measure data validation can become quite chaotic for all. In this post and in a recent OSIS Webinar Series: Quality Measure Data Validation 101, we go back to the basics of quality measure data validation to help keep your health center on track for 2022 reporting. “Going back to the basics is the simplest way to find calm in the chaos.” Author Unknown
What is UDS?

Federally Qualified Health Centers (FQHCs) each year are required to report on a core set of information, including data on patient characteristics, services provided, clinical processes and health outcomes, and much more as part of a standardized reporting system known as the UDS that is defined in section 330 of the Public Health Service Act. Preparing for UDS Reporting “Season”, FQHCs are expected to have a system in place to collect and organize the data accurately, timely, and completely in accordance with Health Resources and Services Administration (HRSA) instructions.
What is data validation?
According to the Methodology for Data Validation, “Data validation is an activity aimed at verifying whether a combination of values is a member of a set of acceptable combinations.” Data Validation is something health centers need to actively be doing on an ongoing basis. The emphasis is on acceptable here because as validation happens, numerators climb as teams work together to get to the goal, and is a good combination as we look at trends and the goals to which we are striving. If the numerator is not where it needs to be, working on getting it to where it should be is one of the reasons the OSIS team does what they do.
What is Quality Measure Data Validation?
Validating healthcare quality measures is an essential component of measuring care using data derived from complex Electronic Health Records (EHRs). Without validation, errors may be substantial and could introduce bias into calculations, for example, certain providers are omitted or inappropriately included in notes and the denominator. Using information from chart reviews can improve queries interactively fashion to validate the numerator data that will ensure our final estimates of care are correct. Validation also helps health centers understand what is in their NextGen universe is what should be shown and captured correctly.
Why do we perform measure data validation?
It has been well-promoted, that the goal is to create a data-driven culture within OSIS and throughout the healthcare industry. If organizations haven’t adopted a data-driven culture, they may be falling behind the curve of where technology is taking us. Looking at the data and using it can determine where you’re at as an organization, what changes need to be made, and celebrate or truly see the impact that health centers are making. Data needs to be incorporated all throughout the culture of your health center. The Center for Care Innovations provides an excellent resource on this topic. Just a few examples are:
Data Governance: The ability to do something with the information obtained. It is important to remember that staff members, processes, and technical tools help transform data into actionable information.
Data Stewardship: Responsibility for the data reported. Taking ownership of the data provided, ensuring accuracy and completeness, and having a designated Data Stewardship are essential in this concept. The Data Stewardship should have confidence in the data, should be able to validate routinely, and will reflect all the work being done.

Concepts to Consider in Any Validation Process
Not everyone is going to have the same processes for quality measure data validation, but it is important to establish workflows and processes for your health center that are ongoing. We’ll go over a few concepts to keep in mind when establishing a process for completing quality measure data validation.
Know Your Measure:
Familiarizing yourself with measure details your health center needs to capture needs to be done on a regular basis. Just being told “We need to report the BMI of all patient’s seen” is not enough information. You need to understand the measure-specific details – age ranges, visit parameters, exclusion/exception criteria, etc. Understanding the "intent of measure" is important; it can help you report a more accurate capture. This can be tricky as measures change, especially in UDS Reporting, so it’s important to understand the measures currently and the changes that may be coming.
Know your Resources:
Familiarity with the resources you have on hand to review your data is essential. Are the tools used last year for reporting up to date for the upcoming year? Is your health center due for a NextGen upgrade? Has the organization adopted new programs that could be utilized to capture data? Has there only been one person working on validation and now there is additional staff onboard that can assist to improve accuracy? Is there an OSIS Solution available that can pull this data for you?
There are a lot of resources to help health centers work smarter. As an OSIS Member, we provide each Member with a Quality Consultant to provide services to help with monthly check-ins to assess your organization’s processes and workflows, where are the pain points, getting updates on changing measures, what the future look like from resources like HRSA or NACHC or any other agencies that we meet with routinely.
Within NextGen HER, there are many resources that help capture the information, to help with mapping for reporting, and tools that help with data capture like the UDS Reporting Console that helps with capturing UDS measures. The console can also be used if you have similar type measures maybe not UDS Specific, but the tool can be used for other types of reporting, based on their parameters.
Engage Fellow Employees:
This is not just your data, it’s the organization’s data. Ensure the team is involved in understanding the measures, vocalize about where documentation occurs, be transparent in where documentation needs to occur to ensure accurate capturing, and share the outcomes or celebrations routinely. Not only should the Validation Steward be reviewing the data often, but that information needs to be shared throughout the organization. When a measure reaches the goal, that’s an all-hands win! If there is a measure that isn’t doing as well, there may be an intervention that needs to happen.
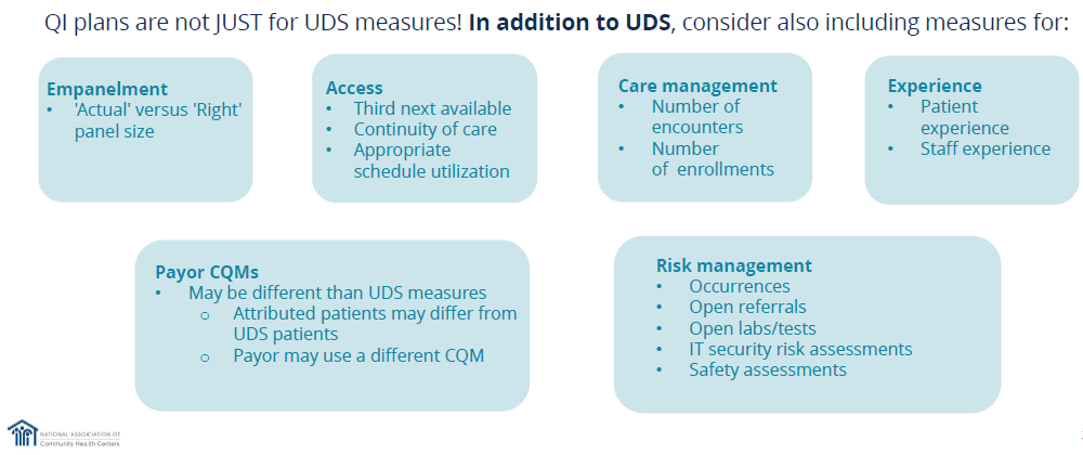
Below, we’d like to highlight some key points of the National Association of Community Health Centers' (NACHC’s) Improvement Strategy they recently revealed. The visual from their presentation highlights crucial aspects of engaging key employees.

Four tactics to follow for quality measure data validation:
Tactic One: Assign a Data Validation Lead
Tactic Two: Set a Schedule
Setting a schedule and working on validation periodically throughout the year is important. This will give your practice a constant overview of trends and adverse measures to adjust in real time before getting to the reporting phase. Data validation can feel very overwhelming when there are so many measures to review. Also, the point of validation is to catch if changes are needed BEFORE the time for reporting. Doing routine audits on a set schedule can help prevent data overload and allows us to stay ahead so changes can be made and monitored over time before the outcome needs to be reported.
Another point NACHC brought up in their Improvement Strategy session was to consider including in your schedule other measures outside of UDS measures. See their recommendations below.
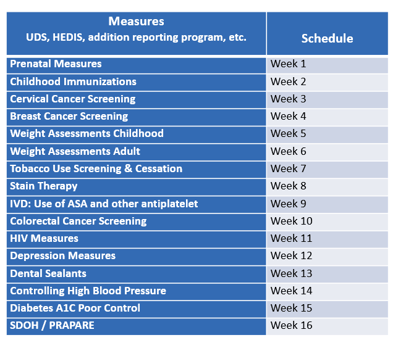
Here is an example of a deep-dive assessment schedule that should be repeated on a quarterly basis to monitor changes over time. After week 16, the schedule will repeat. For other reporting measures, these may be lumped into a set period to combine the efforts. Validations can be clumped together to be more effective as you’re putting together the schedule and adding it to your calendar. Making sure it gets added to your calendar will also help keep your team organized.
Tactic Three: Follow a Standardized Framework
There are a lot of evidence-based procedures on how to do this type of work that can be applied toward quality measure data validation. These resources can be included in the validation to help achieve organizational goals.
- Plan-Do-Study-Act (PDSA) Worksheet and other resources
- Failure Mode and Effect Analysis – a process analysis tool that looks at the means or ways processes might fail and then studies the consequences of those failures.
- Root Cause Analysis (RCA) – RCAs are recommended to identify what happened, why it happened, and determine what changes need to be made. This framework also follows the Performance Improvement Project (PIPs) plan.
Tactic Four: Chart Audits
It is important that the audit team includes both a clinical staff member and an electronic health record analyst who understands where in the EHR the report data is being pulled from. Perform a random sample of the numerator and criteria not met categories to catch potential errors or gain confidence in what is being captured. Auditing is a smart way to detect changes that can impact data, such as upgrades, and keeping track of these changes is key to improving data validation. Make sure you are getting into the charts and in the weeds at the patient level.
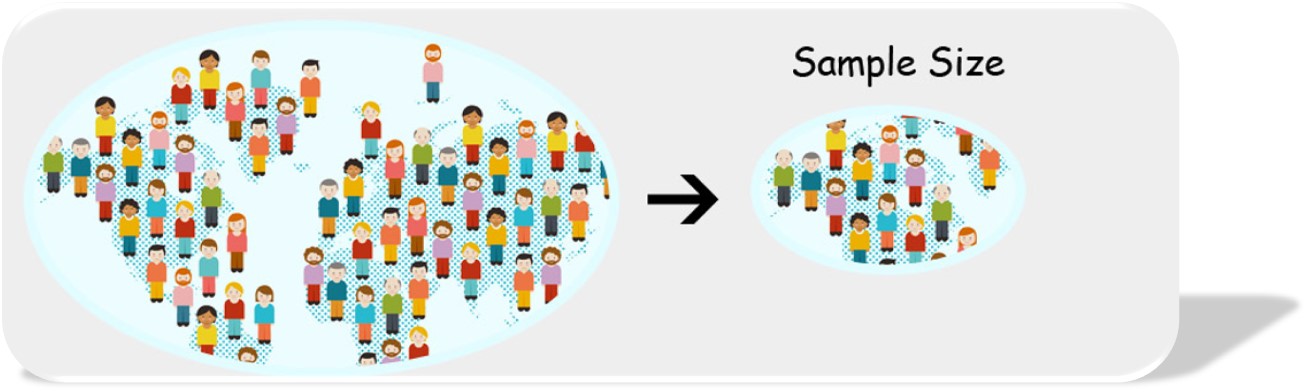
Importantly, keep in mind the sample size for your validation. To feel confident in your accuracy, a good maximum sample size is usually 10% if it does not exceed 1000. Keep in mind, the smaller the sample size, the larger the margin of error.
Central Limit Theorem states a minimal sample size of 30 is often sufficient. This is the logic our OSIS Quality Consultants most often follow. Typically, within 30 charts they can identify issues that may need to be corrected. If the population is much greater than 300 but within 30 reviews and problems are not identified, then a deeper dive is recommended.
To recap some of the basics we went over, here are just some of the questions to consider as you start your reporting for 2022 and prepare for an easier 2023 reporting season.
Review your current Quality Improvement (QI) process and ask yourself…
Does your health center QI strategy support different programs within your organizational goals?- Does it support Value-Based Care (VBC) transformation?
- Are you leveraging data to drive improvement?
- Are you connecting improvement efforts with outcomes you are trying to achieve?
- Do you have set goals for each measure selected for intervention?
- Are you performing optimization or expert reviews to select measures with your team? Are there updates or changes that need to be addressed?
- Are you Identifying themes and recognizing areas of collaboration What is working well in your current QI process and what adjustments may need to be made for you and your team to be more effective in the work that is trying to be achieved? Are there conversations actively happening?
- Does the QI process account for celebrations?
If the answers to the above questions are no, consider going back to the basics with your OSIS Quality team.
UDS Resources: Methodology for Data Validation 1.0
Health Resources and Services Administration
Improvement Strategy, Elevate. National Association of Community Health Center. 11 October 2022.
.png?width=350&height=300&name=Blog%20graphics%20(1).png)